| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Oxidation and Reduction for Medical Students
Related Subjects: |Basic Chemistry for Medicine |Basic Physics for Medicine |Electron Transport Chain |Oxidation and Reduction for Medical Students
Oxidation and Reduction for Senior Medical Students
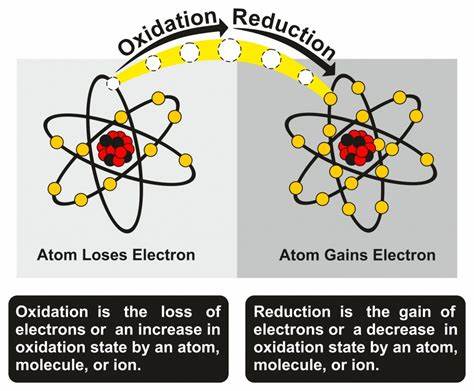
Understanding redox (reduction–oxidation) is foundational to physiology, pathology, pharmacology, and therapeutics. From mitochondrial ATP generation to neutrophil killing, from CYP450 drug metabolism to ischemia–reperfusion injury, redox couples control energy flow and signalling across cells and organs.
Core Concepts (beyond the basics)
- Working definitions
- Oxidation = loss of electrons (↑ oxidation state). Mnemonic: OIL RIG / LEO the lion says GER.
- Reduction = gain of electrons (↓ oxidation state).
- Oxygenation ≠ Oxidation (always): Adding O often coincides with oxidation, but redox is defined by electron transfer/oxidation state—not simply O addition.
- Redox pair (couple): Oxidized form / reduced form (e.g., NAD+/NADH, GSSG/GSH, Cyt c(Fe3+)/Cyt c(Fe2+)). Electrons flow spontaneously from the couple with lower reduction potential to higher.
- Energetics and directionality
- Standard reduction potential E°′ (V) ranks affinity for electrons. Larger E°′ → stronger oxidant.
- Nernst equation links potential to ratio of oxidized:reduced forms; physiological E depends on concentrations and pH.
- Free energy: ΔG = −nFΔE. A positive ΔE (acceptor − donor) drives a negative ΔG (spontaneous). This underpins the electron transport chain (ETC) and oxidative phosphorylation.
Physiologic Redox Systems You Should Know
- NAD+/NADH vs NADP+/NADPH
- NAD+/NADH: Catabolic energy (glycolysis, TCA, β-oxidation) → feeds electrons to ETC for ATP.
- NADP+/NADPH: Reductive biosynthesis and antioxidant defense (fatty acid/cholesterol synthesis, GSH recycling, nitric oxide synthase, CYP450). Major cytosolic source: pentose phosphate pathway (G6PD).
- Glutathione system
- GSH (reduced) detoxifies peroxides via glutathione peroxidase → becomes GSSG (oxidized).
- Glutathione reductase uses NADPH to regenerate GSH. The GSH:GSSG ratio reflects cellular redox status.
- Thioredoxin–peroxiredoxin: Parallel antioxidant system using NADPH to maintain protein thiols in a reduced state and remove H2O2.
- Heme iron redox: Hemoglobin iron cycles Fe2+ (binds O2) ↔ Fe3+ (methemoglobin; cannot bind O2). MetHb reductase (NADH-dependent) restores Fe2+.
Redox in Cellular Respiration (integration)
- Glycolysis: Glyceraldehyde-3-phosphate → 1,3-BPG produces NADH (cytosolic). Shuttle systems move reducing equivalents to mitochondria (malate–aspartate; glycerol-3-phosphate shuttles).
- TCA cycle: Multiple dehydrogenases generate NADH and FADH2.
- ETC: NADH/FADH2 are oxidized; O2 is reduced to H2O. Proton gradient powers ATP synthase. Hypoxia halts Complex IV → ↑NADH/NAD+, ↑lactate:pyruvate ratio.
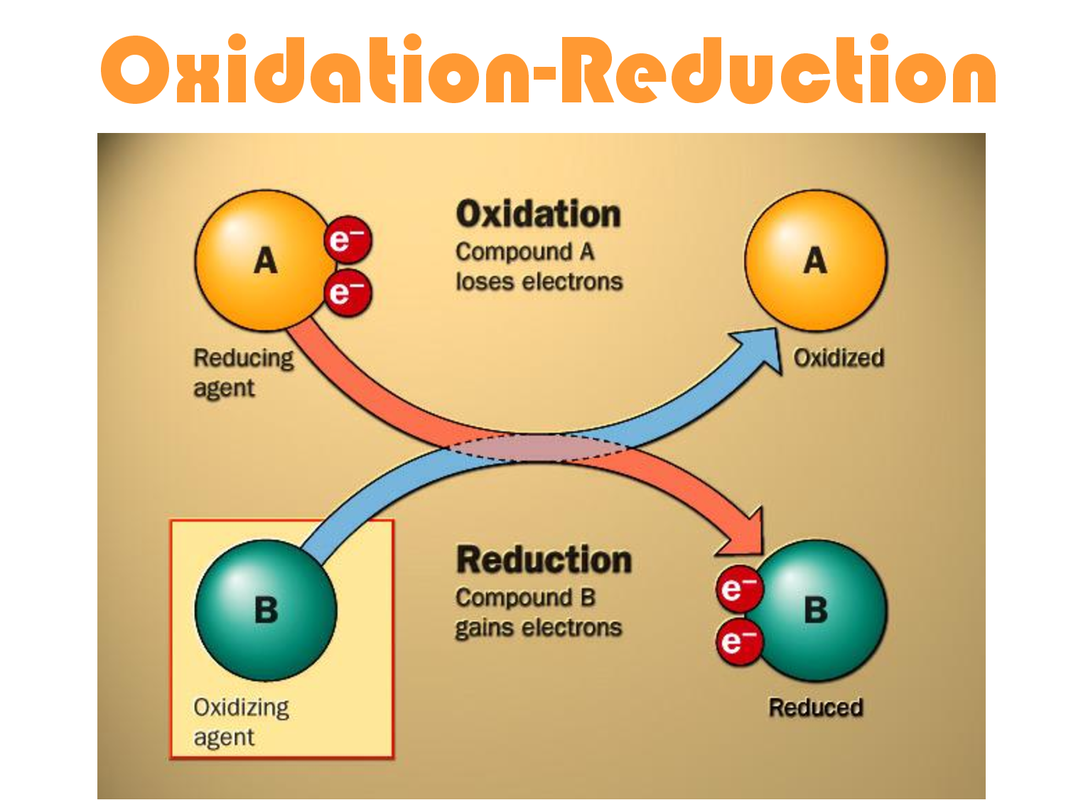
Redox in Detoxification & Xenobiotic Metabolism
- Cytochrome P450 (CYP) monooxygenases (ER membrane):
- Overall: RH + O2 + NADPH + H+ → ROH + H2O + NADP+.
- Phase I oxidation introduces/uncovers polar groups; Phase II conjugation (UGT, SULT, GST) increases solubility.
- Neutrophil oxidative burst:
- NADPH oxidase: O2 → O2•− (superoxide) → H2O2 (SOD) → HOCl (myeloperoxidase). Critical for killing catalase-positive organisms.
Reactive Oxygen Species (ROS) & Signalling
- Sources: ETC Complex I/III leak, CYP450, peroxisomes, xanthine oxidase, NOX enzymes.
- Species: O2•−, H2O2, •OH. H2O2 also acts as a signalling oxidant (reversible cysteine sulfenylation) modulating kinases/phosphatases.
- Defense: SOD, catalase, GPx/Prx, GSH/GST, thioredoxin. Dysfunction → oxidative damage to lipids (MDA, 4-HNE), proteins, DNA (8-oxo-dG).
High-Yield Clinical Correlates
- G6PD deficiency (hemolysis):
- ↓NADPH → impaired GSH regeneration → RBCs vulnerable to oxidants (fava beans, infections, drugs like primaquine, dapsone, nitrofurantoin).
- Findings: Bite cells, Heinz bodies.
- Methemoglobinemia:
- Oxidants (nitrates, local anesthetics like benzocaine) convert Hb Fe2+ → Fe3+.
- Chocolate-brown blood, cyanosis refractory to O2, normal PaO2, low SpO2.
- Treatment: Methylene blue (accepts electrons from NADPH to reduce MetHb); avoid in G6PD deficiency (risk hemolysis).
- Acetaminophen toxicity:
- CYP generates NAPQI (electrophile) → detoxified by GSH. Overdose depletes GSH → hepatic necrosis.
- N-acetylcysteine replenishes GSH and enhances non-toxic conjugation.
- Ischemia–reperfusion injury:
- Hypoxia stalls ETC; reperfusion floods O2, mitochondria and xanthine oxidase produce ROS → lipid peroxidation, mPTP opening, cell death. Antioxidant systems and ischemic preconditioning mitigate.
- Chronic Granulomatous Disease (CGD):
- NOX2 defect → impaired oxidative burst → recurrent infections with catalase-positive organisms (S. aureus, Serratia, Nocardia, Aspergillus).
- Abnormal DHR flow cytometry test; prophylactic antibiotics/antifungals; IFN-γ in some cases.
- Alcohol metabolism:
- ADH/ALDH generate excessive NADH → drives lactate formation (↑lactate:pyruvate), inhibits β-oxidation, promotes fatty liver (↑glycerol-3-phosphate → TG synthesis).
- Cyanide/CO poisoning:
- Inhibit Complex IV (Cyt c oxidase) → block O2 utilization (histotoxic hypoxia), ↑NADH/NAD+, rapid ATP failure. Cyanide antidotes induce MetHb (bind CN⁻) + thiosulfate; CO binds Hb & Complex IV.
- Redox-cycling drugs/toxins:
- Doxorubicin generates ROS (cardiotoxicity); paraquat undergoes redox cycling in lung → severe oxidative injury.
Numbers, Ratios, and Bedside Markers
- Lactate:pyruvate ratio reflects cytosolic NADH/NAD+ (↑ in hypoxia, sepsis, ethanol excess).
- Acetoacetate:β-hydroxybutyrate reflects mitochondrial NADH/NAD+ status (↑β-HB in high NADH states).
- GSH:GSSG ratio as a cellular redox health indicator (lower ratio = oxidative stress).
Redox in Therapeutics & Research
- Antioxidants (e.g., vitamin C/E) can be helpful in defined deficiencies but may blunt adaptive redox signalling if misused.
- NAD+-boosting strategies (niacin, NR/NMN) and mitochondrial-targeted antioxidants (MitoQ) are areas of active study.
- Redox-sensitive signalling (Keap1–Nrf2) regulates cytoprotective gene programs (phase II enzymes, GSH synthesis).
Summary
Redox chemistry is the currency of biological energy and defence. Electron flow from reduced substrates to oxidants powers ATP synthesis, shapes biosynthesis via NADPH, and modulates signalling through controlled ROS. Clinically, redox principles explain hemolysis in G6PD deficiency, methemoglobinemia therapy, acetaminophen antidotes, reperfusion injury, and the action/toxicity of many drugs. Mastering redox couples, potentials (ΔE), and their link to ΔG equips you to interpret metabolic states, choose therapies, and anticipate complications.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
