| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Acute Myeloblastic Leukaemia (AML)
Related Subjects: | Leukaemias in General | Acute Promyelocytic Leukaemia | Acute Myeloblastic Leukaemia (AML) | Acute Lymphoblastic Leukaemia (ALL) | Chronic Lymphocytic Leukaemia (CLL) | Chronic Myeloid Leukaemia (CML) | Hairy Cell Leukaemia | Differentiation Syndrome | Tretinoin (All-trans-retinoic acid (ATRA)) | Haemolytic Anaemia | Immune (Idiopathic) Thrombocytopenic Purpura (ITP)
Acute Myeloid Leukaemia (AML) is an aggressive haematological malignancy 💉 arising from clonal proliferation of immature myeloid precursors (myeloblasts). These abnormal cells accumulate in the bone marrow 🦴, replacing normal haematopoiesis and spilling into blood and tissues. AML mainly affects older adults 👵👴, progresses rapidly, and requires urgent treatment. Classic features include anaemia, infections, bleeding, bone pain, and hepatosplenomegaly.
ℹ️ About
- AML is a neoplastic clone of immature myeloid precursors 🧬
- Auer rods (needle-like inclusions) are characteristic 🔬
- Can be primary or secondary (e.g. post-myelodysplasia, post-chemo/radiation) ⚠️
Types
- Primary AML: arises de novo
- Secondary AML: follows MDS, myeloproliferative disease, or cytotoxic therapy 🎗️
🧬 Aetiology
- Clonal proliferation with impaired differentiation → marrow packed with blasts 🚨
- Suppression of normal cells → anaemia 😴, thrombocytopenia 🩸, neutropenia 🤒
- Blast infiltration → gum hypertrophy, skin nodules, CNS infiltration
Genetic Translocations
- t(8;21) → favourable prognosis ✅
- t(15;17) → Acute Promyelocytic Leukaemia (APL, M3 subtype), high risk of DIC ⚠️ but excellent response to ATRA 🎯
- inv(16) → favourable group 📊
Risk Factors
- Age > 60 years (median age ~70) 👵
- Genetic predispositions: Down syndrome (esp. M7, 400× risk), Fanconi anaemia
- Environmental: benzene ☣️, ionising radiation ☢️
- Therapy-related AML: post-alkylators, topoisomerase II inhibitors 💊
- Pre-existing: MDS, myeloproliferative neoplasms
FAB Classification (M0–M7)
- M0: Minimally differentiated AML, CD13+/CD33+
- M1: Myeloblastic, no maturation
- M2: Myeloblastic with maturation (Auer rods present) 🔬
- M3: Acute Promyelocytic Leukaemia (APL) – t(15;17), ATRA-sensitive ⭐
- M4: Myelomonocytic → gum infiltration common
- M5: Monocytic → gum/CNS infiltration 🧠
- M6: Erythroleukaemia
- M7: Megakaryoblastic AML → associated with Down syndrome
AML M3
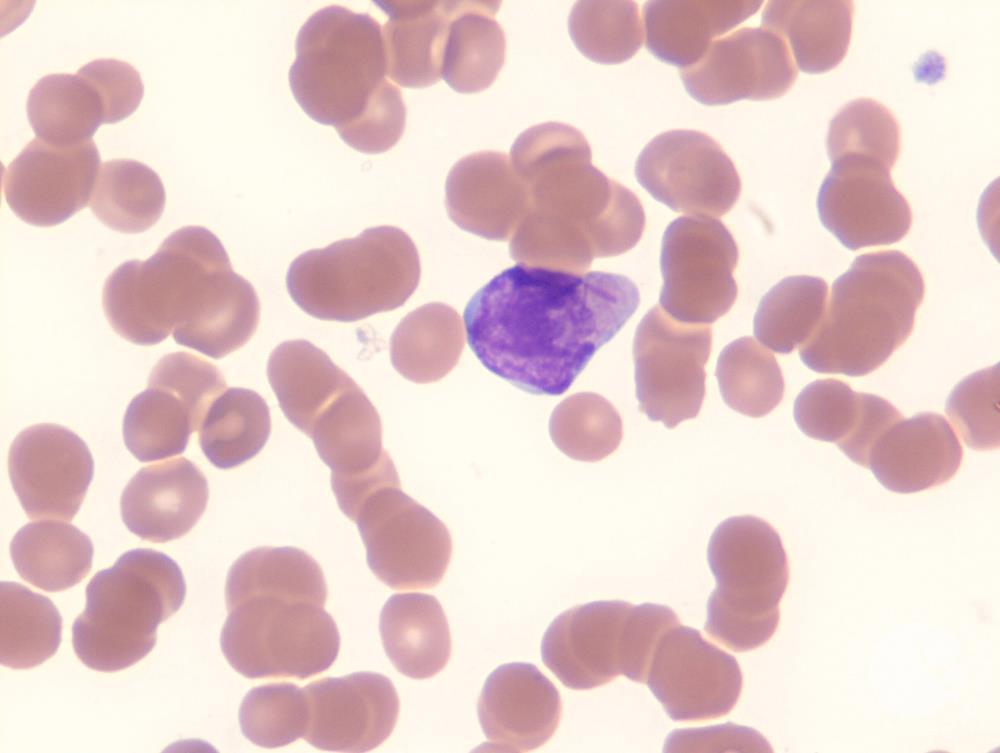
WHO Classification
- AML with recurrent genetic abnormalities (e.g. t(8;21), inv(16), t(15;17))
- AML with multilineage dysplasia (post-MDS)
- Therapy-related AML (poor prognosis) ❌
- AML not otherwise specified
🩺 Clinical Features
- Marrow failure:
- Anaemia → fatigue, pallor 😴
- Thrombocytopenia → bruising, bleeding, petechiae 🩸
- Neutropenia → recurrent infections 🤒
- Infiltration: hepatosplenomegaly, lymphadenopathy, gingival hypertrophy, skin nodules
- Special: DIC risk in APL (M3) ⚡
- Myeloblastomas (chloromas) → extramedullary masses
🔎 Investigations
- FBC: Pancytopenia, circulating blasts
- Peripheral smear: Auer rods (needle-like azurophilic inclusions) 🔬
- Bone marrow biopsy: >20% blasts (WHO criterion)
- Cytogenetics: risk stratification (favourable vs poor)
- Other: U&E, LDH, uric acid (tumour lysis risk ⚠️)
- Coagulation screen: DIC risk in M3
💊 Management
- Supportive: transfusions 🩸, antibiotics/antifungals 💊, central line, tumour lysis prophylaxis (hydration + allopurinol/rasburicase)
- Induction chemotherapy: “7+3” regimen (cytarabine 7 days + anthracycline 3 days) 💉
- Consolidation: high-dose cytarabine or SCT in high-risk patients
- APL (M3): ATRA + arsenic trioxide (or anthracycline). Watch for differentiation syndrome ⚠️
- Targeted agents:
- FLT3 inhibitors (midostaurin) 🎯
- IDH1/2 inhibitors
- Gemtuzumab ozogamicin (anti-CD33) in certain cases
- Stem cell transplant: for high-risk or relapsed disease 🌱
References
🧑⚕️ Case Examples — Acute Myeloblastic Leukaemia (AML)
- Case 1 (Pancytopenia presentation): 🩸 A 68-year-old man presents with progressive fatigue, recurrent infections, and easy bruising. Exam shows pallor, petechiae, and mild hepatosplenomegaly. Bloods: Hb 6.8 g/dL, WCC 1.5 × 10⁹/L, platelets 30 × 10⁹/L. Peripheral smear: blasts with Auer rods. Analysis: Bone marrow failure due to malignant myeloblast proliferation. Diagnosis: Acute Myeloblastic Leukaemia. Management: Bone marrow biopsy confirms AML. Induction chemotherapy (e.g., cytarabine + anthracycline), supportive transfusions, infection prophylaxis. Allogeneic stem cell transplant considered if poor prognosis cytogenetics.
- Case 2 (Hyperleukocytosis + leukostasis): 🫁 A 45-year-old woman presents with acute dyspnoea, headache, and confusion. Bloods: WCC 120 × 10⁹/L, blasts on film. Chest X-ray: diffuse pulmonary infiltrates. Analysis: Symptomatic hyperleukocytosis → leukostasis (microvascular occlusion). Life-threatening emergency. Diagnosis: AML with hyperleukocytosis and leukostasis. Management: ICU admission, urgent cytoreduction (hydroxycarbamide or leukapheresis), followed by induction chemotherapy. Supportive care with fluids and tumour lysis prophylaxis (allopurinol/rasburicase).
- Case 3 (Acute Promyelocytic Leukaemia – APML): ⚠️ A 32-year-old woman presents with mucosal bleeding, bruising, and petechiae. Bloods: Hb 9.2 g/dL, WCC 2.8 × 10⁹/L, platelets 20 × 10⁹/L, prolonged PT/APTT, low fibrinogen. Blood film: abnormal promyelocytes with Auer rods. Analysis: APML (AML M3) associated with disseminated intravascular coagulation (DIC). Medical emergency with high early mortality. Diagnosis: Acute Promyelocytic Leukaemia (t(15;17) translocation, PML-RARA fusion). Management: Immediate initiation of all-trans retinoic acid (ATRA) + arsenic trioxide, aggressive clotting support, haematology urgent referral. Prognosis excellent if treated early.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
