| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Electron Transport Chain
Related Subjects: |Basic Chemistry for Medicine |Basic Physics for Medicine |Electron Transport Chain |DNA replication |DNA structure in Nucleus |Cell Cycle |Mitosis and Meiosis |Ribosomes |Microtubules |Mitochondria |Smooth and Rough Endoplasmic Reticulum
🚀 The Electron Transport Chain (ETC) is the final stage of cellular respiration, embedded in the inner mitochondrial membrane. Its whole point is to convert the energy in electrons (from NADH and FADH₂) into a proton-motive force and then into ATP via oxidative phosphorylation. Without the ETC, cells cannot efficiently regenerate NAD⁺/FAD, aerobic metabolism stalls, and ATP yield collapses. In short: ETC → proton gradient → ATP = life’s energy economy ⚡️.
📍 Location
🎯 Why Cells Bother with the ETC (Key Outcomes)
- ATP production (oxidative phosphorylation) 🔋:
- Electron flow → H⁺ pumping → Δp (proton-motive force) → ATP synthase activity.
- Rule of thumb: ~2.5 ATP per NADH (Complex I entry) & ~1.5 ATP per FADH₂ (Complex II entry).
- Redox balance 🔁: Re-oxidizes NADH → NAD⁺ and FADH₂ → FAD so glycolysis, β-oxidation, and the TCA cycle can continue.
- Efficient energy capture 🧠: Stepwise transfers minimize heat loss and maximize ATP per fuel molecule (glucose, fatty acids, ketones).
- Metabolic control 🎚️: Flux adapts to ADP, substrate availability, and O₂—matching ATP output to demand (aerobic “cruise control”).
- Thermogenesis (when uncoupled) 🔥: In brown fat, UCP1 allows H⁺ leak, dissipating energy as heat (cold adaptation).
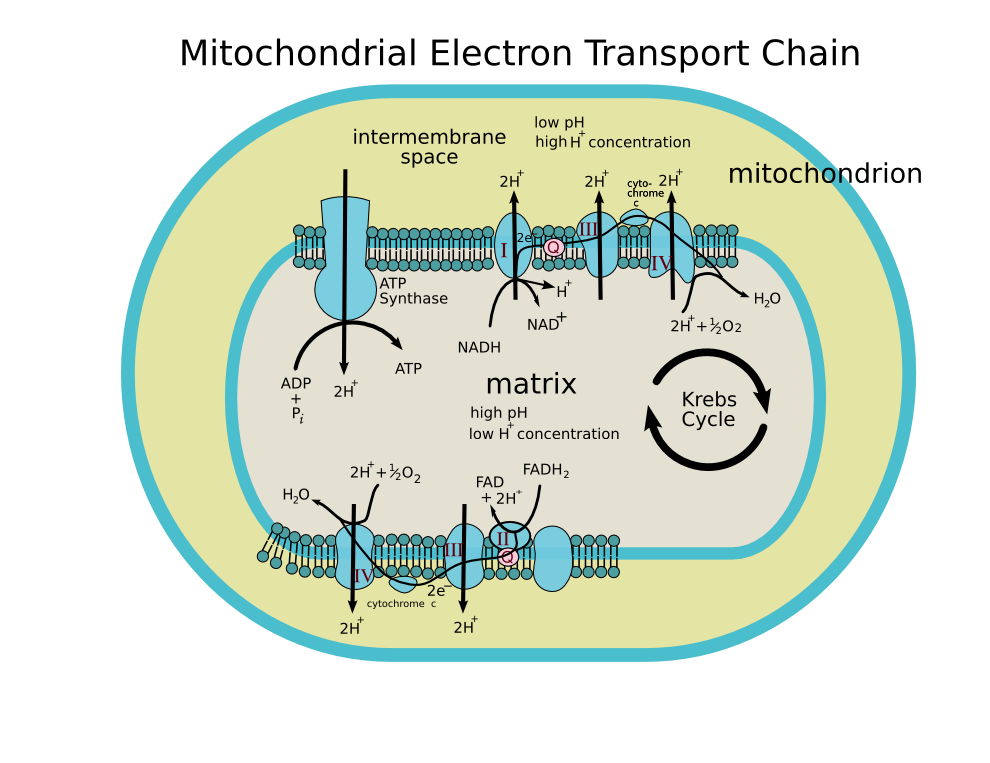
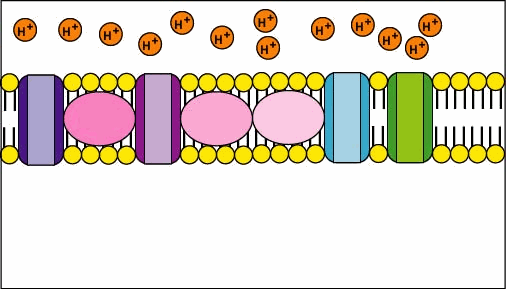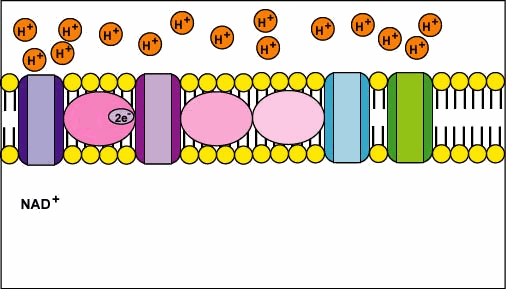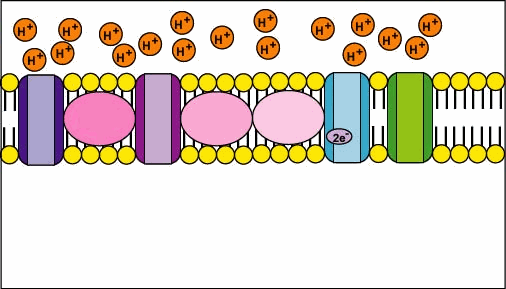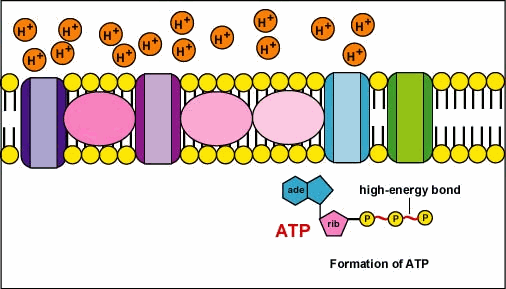
🧩 Components of the Electron Transport Chain
- Complex I (NADH:Ubiquinone Oxidoreductase) 🧱
- Accepts electrons from NADH and passes them to ubiquinone (Q).
- Pumps H⁺ from matrix → intermembrane space (start building the gradient).
- Complex II (Succinate:Ubiquinone Oxidoreductase) 🔗
- Receives electrons from succinate via FADH₂; transfers to Q.
- Does not pump protons but feeds the chain with electrons.
- Ubiquinone (Coenzyme Q) 🛶
- Lipid-soluble “ferry” shuttling electrons from Complex I/II to Complex III within the membrane.
- Complex III (Cytochrome bc1) ⚙️
- Runs the Q-cycle: transfers electrons from QH₂ to cytochrome c.
- Pumps additional H⁺ to the intermembrane space.
- Cytochrome c 🧪
- Small, water-soluble carrier on the outer surface of the inner membrane; delivers electrons to Complex IV.
- Complex IV (Cytochrome c Oxidase) 🧲
- Accepts electrons from cytochrome c and reduces O₂ → H₂O (terminal step).
- Pumps H⁺; this final step keeps the entire chain flowing.
- ATP Synthase (Complex V) 🌀
- Not an electron carrier; the turbine that converts Δp into ATP as H⁺ returns to the matrix.
🔬 Mechanism of the Electron Transport Chain
- Electron Transfer ⚡️
- NADH (Complex I) and FADH₂ (Complex II) donate electrons → carriers → Complex IV.
- Energy released stepwise—captured as proton pumping work.
- Proton Pumping 🧯
- Complexes I, III, IV pump H⁺ to intermembrane space → build proton-motive force (Δp).
- ATP Synthesis 🔩
- H⁺ falls down its gradient through ATP synthase → conformational changes → ADP + Pi → ATP.
🧮 The Proton-Motive Force (Δp)
- Two components: membrane potential (Δψ) and pH gradient (ΔpH).
- Formal relation: Δp = Δψ − (2.303·RT/F)·ΔpH (drives H⁺ back via ATP synthase).
- ETC builds Δp; ATP synthase spends Δp. Uncouplers collapse Δp → ↑O₂ use, ↓ATP, ↑heat.
🌬️ Role of Oxygen
- O₂ is the terminal electron acceptor at Complex IV.
- Electrons + H⁺ + O₂ → H₂O 💧; this removal of electrons keeps the “traffic” flowing through the chain.
- No O₂ (hypoxia) → chain stalls → NADH/FADH₂ accumulate → NAD⁺/FAD scarce → TCA and β-oxidation slow → ATP production plummets.
🧯 Efficiency, ROS, and Uncoupling
- Efficiency 📈: Not all energy becomes ATP; some becomes heat (useful for thermoregulation).
- ROS formation 🧨: Small electron leaks (Complex I/III) → superoxide. Detoxed by SOD, catalase, glutathione peroxidase systems.
- Coupling vs. Uncoupling:
- Tightly coupled 🔒: High ATP yield; O₂ consumption scales with ADP (acceptor control).
- Uncoupled 🔓: H⁺ leaks (UCP1, DNP, FCCP) → ↑O₂ use, ↓ATP, ↑heat (thermogenesis/overheating risk).
⛔ Classic Inhibitors & Where They Act
- Complex I 🧱: Rotenone, amobarbital (block NADH→Q electron flow).
- Complex III ⚙️: Antimycin A (blocks QH₂ oxidation → cyt c reduction).
- Complex IV 🧲: Cyanide (CN⁻), carbon monoxide (CO), azide (N₃⁻) — stop O₂ reduction → arrest respiration.
- ATP Synthase 🌀: Oligomycin (blocks H⁺ channel) → Δp rises but ATP synthesis halts.
- Uncouplers 🔥: DNP, FCCP (collapse Δp; electrons still flow, ATP falls, heat rises).
🧭 Integration with Metabolism (Big-Picture Links)
- Glycolysis → NADH (cytosolic) shuttled to mitochondria (malate-aspartate or glycerol-phosphate shuttles) → ETC.
- TCA cycle → NADH/FADH₂ (mitochondrial) → ETC (primary electron supply under aerobic conditions).
- β-oxidation of fatty acids → lots of FADH₂/NADH → high ATP yield (ETC-dependent).
- ADP supply (work/exercise) speeds ETC (acceptor control); phosphate availability and substrate supply tune flux.
🩺 Clinical Relevance
- Mitochondrial diseases 🧬: ETC defects → myopathy, exercise intolerance, neuro symptoms; maternal inheritance common (mtDNA).
- Hypoxia/Ischemia 🫁🫀: O₂ limitation stalls Complex IV → ATP crisis, ion-pump failure, Ca²⁺ overload, ROS on reperfusion → cell death.
- CO poisoning ☠️: CO binds Complex IV (and Hb), blocking O₂ use despite normal O₂ content in air.
- Drugs/Toxicology 💊:
- Linezolid (mitochondrial translation), antiretrovirals (mtDNA polymerase γ) → secondary mitochondrial dysfunction.
- High mitochondrial metformin levels can modestly inhibit Complex I → ↓hepatic gluconeogenesis (AMPK-linked).
- ROS-linked disorders 🌩️: Neurodegeneration, aging biology, cancer signaling—balance between necessary signaling ROS vs. damaging excess.
🧪 Quick Numbers & Nuggets
- Inner membrane is highly impermeable; cardiolipin helps maintain the proton seal.
- Δψ typically ~150–180 mV; total Δp corresponds to ~200 mV (context-dependent).
- Each full rotation of ATP synthase’s Fo/F1 makes ~3 ATP (exact H⁺/ATP ratio depends on c-ring stoichiometry).
- Per glucose aerobically: ~30–32 ATP total (pathway- and tissue-dependent assumptions).
🧭 Learning Aids (Mental Models)
- “Dams and turbines” 💧⚙️: Complexes I/III/IV build the water behind the dam (H⁺ gradient). ATP synthase is the turbine turning flow into electricity (ATP).
- “Redox conveyor belt” 🛤️: NADH/FADH₂ hand electrons down the belt to O₂; each handoff funds proton pumping.
- “Clutch and gearbox” 🚗: ADP is the “clutch” that engages respiration—more ADP, more ETC flux and ATP delivery.
🧵 Summary (The Take-Home)
Purpose 🎯: Use high-energy electrons from nutrient oxidation to build a proton gradient that powers ATP synthase.
Payoff 💰: High-yield ATP and continuous regeneration of NAD⁺/FAD for upstream metabolism.
Trade-offs ⚖️: Potential ROS and heat—usually controlled or harnessed (e.g., thermogenesis).
Bottom line ✅: The ETC is biology’s premier energy-conversion turbine—turning food-derived electrons and oxygen into the ATP that runs nearly everything else.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
