| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Basic Chemistry for Medicine
Related Subjects: | Basic Chemistry for Medicine
🧪 Basic Chemistry for Medicine
Understanding basic chemistry is essential for medical professionals 🩺. It underpins physiology, drug interactions, and laboratory diagnostics. This guide introduces the fundamental concepts most relevant to medicine, with clinical anchors (ABGs, IV fluids, enzymes, and pharmacology) to make the science stick.
⚛️ Atoms and Molecules
- Atoms:
- Smallest unit of matter, with a nucleus (protons ➝ +, neutrons ➝ neutral) surrounded by electrons (−).
- Electrons occupy orbitals/shells; their configuration (valence electrons) drives chemical reactivity and bonding.
- Isotopes: same element, different neutrons (e.g., 99mTc in nuclear medicine).
- Molecules:
- Formed by bonding between atoms.
- Types of bonds:
- Ionic: electron transfer, electrostatic attraction (e.g., NaCl, many electrolytes in plasma).
- Covalent: electron sharing (e.g., H2O, amino acids, glucose); may be polar (unequal sharing) or non-polar.
- Hydrogen bonds: weak individually but crucial for DNA base pairing and protein secondary structure.
- Van der Waals / hydrophobic interactions: stabilize protein folding and lipid bilayers.
- Stereochemistry:
- Chirality: mirror-image isomers (enantiomers) can have different pharmacologic effects (e.g., S- vs R-enantiomers).
- Conformation matters for receptor binding and enzyme activity.
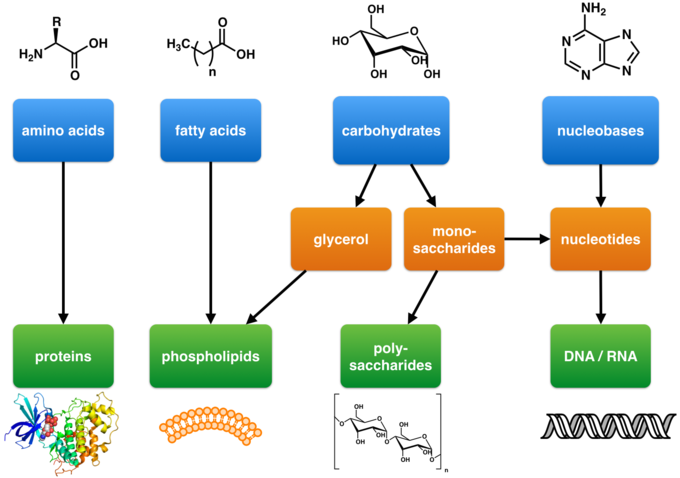
🧷 Chemical Bonds in Biology (clinical angles)
- Peptide bonds link amino acids → proteins; hydrolysed by proteases.
- Glycosidic bonds link sugars → glycogen, starch; broken by amylases.
- Phosphodiester bonds in DNA/RNA backbones; target of nucleases and many chemotherapeutics.
- Disulfide bonds stabilize extracellular proteins (e.g., insulin A–B chains).
⚡ Chemical Reactions
- Types:
- Combination: A + B → AB
- Decomposition: AB → A + B
- Displacement: AB + C → AC + B
- Redox: electron transfer (e.g., Fe2+ → Fe3+ in haemoglobin oxidation; reactive oxygen species in sepsis/ischemia-reperfusion).
- Acid–base: proton transfer (buffers, ABGs).
- Hydrolysis/Condensation: water in/out (peptide formation/breakdown).
- Reaction Rates (Kinetics):
- Increase with ↑ temperature, ↑ concentration, ↑ surface area, and catalysts (enzymes).
- Enzyme kinetics: Michaelis–Menten — v = (Vmax[S])/(Km+[S]).
- Inhibition:
- Competitive: ↑Km, same Vmax (overcome by ↑[S]).
- Non-competitive: ↓Vmax, same Km.
- Irreversible: enzyme inactivated (e.g., aspirin on COX-1).
- Chemical Equilibrium:
- Reversible reactions reach equilibrium (Keq constant at given T).
- Le Châtelier’s principle: system shifts to oppose changes (e.g., CO2 retention drives H+ via carbonic anhydrase).
🔥 Thermodynamics (why reactions happen)
- ΔG (Gibbs free energy) = ΔH − TΔS. Negative ΔG → spontaneous.
- ATP: couples exergonic to endergonic processes; central to metabolism and active transport (e.g., Na⁺/K⁺-ATPase).
- Oxidative phosphorylation: redox energy → proton gradient → ATP synthase.
💧 Solutions & Concentrations
- Solutions: homogeneous mixtures of solute in solvent (e.g., glucose in plasma).
- Concentration Units:
- Molarity (mol/L) — used for many lab reagents.
- Molality (mol/kg solvent) — temperature independent.
- % solutions (w/v, v/v) — e.g., 0.9% NaCl = 0.9 g/100 mL.
- mEq/L for ions: mEq = mmol × valence (Ca2+ = 2 mEq/mmol).
- Dilutions: C1V1 = C2V2 (drug prep, lab assays).
🌊 Osmolarity, Osmolality & Tonicity (IV fluids)
- Osmolarity = osmoles/L; Osmolality = osmoles/kg (clinically preferred).
- Serum osmolality (calc) ≈ 2[Na⁺] + glucose/18 + urea/2.8 (mg/dL units) or 2[Na⁺] + glucose/5.6 + urea/2.8 (mmol/L units).
- Osmolar gap = measured − calculated (↑ with toxic alcohols).
- Tonicity (effective osmolality): determines water movement across cell membranes.
- 0.9% NaCl ~ isotonic; 5% Dextrose becomes hypotonic after metabolism; hypertonic saline draws water out of cells (raised ICP management).
🧪 Acids, Bases & pH
- Acids: release H+ (e.g., HCl in stomach). Bases: accept H+/release OH−.
- pH scale: 0–14 (7 neutral).
- Blood pH: tightly regulated at 7.35–7.45.
- Acidosis: <7.35, Alkalosis: >7.45.
- Henderson–Hasselbalch (bicarbonate buffer): pH = 6.1 + log10(HCO3− / (0.03 × pCO2)).
- Clinical: Respiratory (pCO2) vs metabolic (HCO3−) components → ABG interpretation and compensation.
🛡️ Buffers
- Prevent large pH changes by absorbing H+/OH−.
- Examples:
- Bicarbonate (extracellular; lungs & kidneys regulate components) — key in respiratory/metabolic acidosis/alkalosis.
- Phosphate (intracellular/renal tubular buffer).
- Protein buffers (e.g., haemoglobin binds H+ and CO2).
🧬 Biomolecules
- Carbohydrates:
- Monosaccharides (glucose, fructose), disaccharides (sucrose, lactose), polysaccharides (glycogen).
- Glycolysis → ATP + pyruvate; gluconeogenesis (liver) → glucose; glycogen synthesis/breakdown (insulin/glucagon-regulated).
- Proteins:
- Amino acids → peptides → proteins; levels of structure: primary, secondary (α-helix/β-sheet), tertiary, quaternary.
- Functions: enzymes, receptors, transport (albumin), antibodies, clotting factors.
- Lipids:
- Triglycerides (energy), phospholipids (membranes), cholesterol (membranes, steroid hormones, bile acids).
- β-oxidation of fatty acids → acetyl-CoA; ketone bodies in fasting/DKA.
- Nucleic acids:
- DNA (genetic code), RNA (mRNA, tRNA, rRNA); transcription/translation → protein synthesis.
- Targets of antivirals/chemotherapy (polymerases, nucleoside analogues).
💊 Pharmacochemistry Essentials
- Ionisation & pH: weak acids/bases cross membranes in their non-ionised form (Henderson–Hasselbalch for drugs).
- Protein binding (e.g., to albumin) affects free (active) drug concentration.
- Solubility: lipophilic drugs cross BBB; hydrophilic stay intravascular/extracellular (relevant to volume of distribution).
- Redox metabolism (CYP450) → phase I/II reactions; enzyme induction/inhibition → interactions.
🧮 Quick Clinical Calculations
- Anion gap = Na+ − (Cl− + HCO3−) (normal ~8–12 without K⁺). ↑ in DKA, lactic acidosis, toxins.
- Corrected calcium (if hypoalbuminaemia): Cacorr ≈ Cameas + 0.02 × (40 − albumin g/L).
- Serum osmolality (calc) (mmol/L) ≈ 2[Na⁺] + glucose/5.6 + urea/2.8.
- Dilution: C1V1 = C2V2 (e.g., prepare 1 L of 1 mol/L solution from 5 mol/L stock → V1 = 200 mL).
📌 Clinical Summary
✅ Chemistry explains physiology: acid–base in sepsis/respiratory failure, redox in oxidative stress, and osmolarity/tonicity in IV fluids and cerebral oedema.
✅ Buffers + pH drive ABG interpretation; bicarbonate/pCO2 ratio is the core mental model.
✅ Enzymes (kinetics, inhibition) and ionisation/solubility clarify drug selection, dose, and interactions.
✅ Quick calcs (anion gap, osmolality, corrected calcium, dilutions) are everyday clinical tools.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
