| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Arterial blood gas sampling
📌 Note: ABG sampling, particularly from the radial artery, can be painful. Offer 1 ml of 1% lidocaine at the puncture site to reduce pain without altering results.
Indications
- Assessment of respiratory failure and guiding therapy.
- Post-cardiac arrest management (baseline and ongoing monitoring).
- Acid–base assessment in critically unwell patients.
What ABG Provides
- pH (acid–base balance).
- Partial pressure of oxygen (PaO₂).
- Partial pressure of carbon dioxide (PaCO₂).
- Bicarbonate (HCO₃⁻).
- Base excess and calculated anion gap.
Preparation
- Wait 15–20 minutes after any O₂ therapy change for steady state.
- Explain procedure, risks (pain, bleeding, thrombosis, infection).
- Gain consent. Consider chaperone.
Contraindications
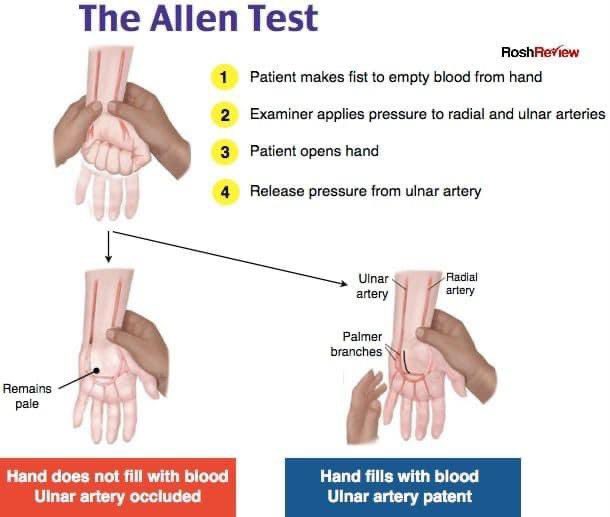
- Poor collateral circulation (check with modified Allen test).
- Severe coagulopathy or anticoagulation (relative).
- Severe peripheral vascular disease.
Equipment
- 2–3 ml heparinised syringe with cap.
- 20–22G needle (radial); larger for femoral access.
- Antiseptic wipes, gauze, tape, sterile gloves.
- Sharps bin.
Procedure
- Wash hands, wear gloves, explain to patient.
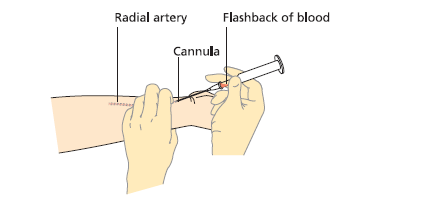
- Identify radial artery. Perform modified Allen test.
- Position wrist extended, palm up. Palpate pulse.
- Clean site with antiseptic.
- Expel excess heparin from syringe. Insert needle at 30–45° (bevel up).
- Bright red blood should pulsate into syringe under pressure.
- Obtain 1–3 ml, then withdraw needle and apply firm pressure ≥5 min (longer if anticoagulated).
- Cap syringe, remove air bubbles, send sample rapidly (on ice if delay).
- Document FiO₂ at time of sampling.
Arterial vs Venous Blood
- Arterial: bright red, fills syringe under pressure.
- Venous: darker, slow flow, may require suction.
Normal Ranges
- pH: 7.35 – 7.45
- PaO₂: 11 – 13 kPa (82 – 97 mmHg)
- PaCO₂: 4.7 – 6.0 kPa (35 – 45 mmHg)
- HCO₃⁻: 22 – 26 mmol/L
- Base excess: –2 to +2
- Anion gap: 8 – 12 (without K⁺)
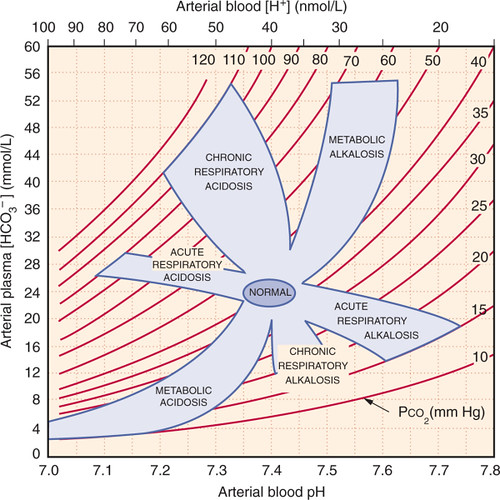
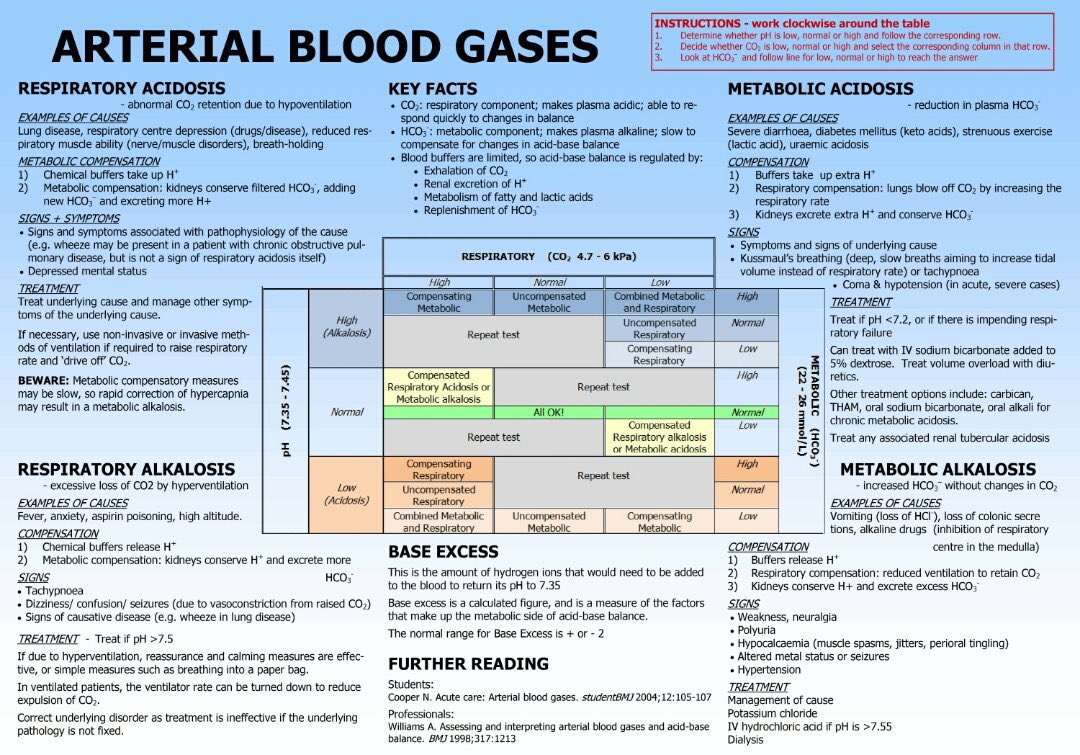
Stepwise Interpretation
- Check pH: acidaemia (<7.35) or alkalaemia (>7.45)?
- Identify primary process:
- Low HCO₃⁻ → metabolic acidosis.
- High HCO₃⁻ → metabolic alkalosis.
- High PaCO₂ → respiratory acidosis.
- Low PaCO₂ → respiratory alkalosis.
- Check compensation:
- Metabolic acidosis → Winter’s formula: PaCO₂ ≈ 1.5 × HCO₃⁻ + 8 (±2).
- Metabolic alkalosis → PaCO₂ ≈ 0.7 × (HCO₃⁻ – 24) + 40.
- Acute resp. acidosis: HCO₃⁻ ↑ ~1 per 10 PaCO₂ ↑.
- Chronic resp. acidosis: HCO₃⁻ ↑ ~3–4 per 10 PaCO₂ ↑.
- Acute resp. alkalosis: HCO₃⁻ ↓ ~2 per 10 PaCO₂ ↓.
- Chronic resp. alkalosis: HCO₃⁻ ↓ ~4–5 per 10 PaCO₂ ↓.
- If metabolic acidosis: calculate anion gap.
- High AG → DKA, lactic acidosis, uraemia, toxins (methanol, ethylene glycol, salicylates).
- Normal AG → diarrhoea, renal tubular acidosis, saline excess.
- Match to clinical context.
Common Patterns & Causes
- Metabolic acidosis: DKA, sepsis/lactate, renal failure, diarrhoea.
- Metabolic alkalosis: vomiting, NG losses, diuretics, hyperaldosteronism.
- Respiratory acidosis: COPD exacerbation, opiates, severe asthma, neuromuscular disease.
- Respiratory alkalosis: anxiety, sepsis, pregnancy, pulmonary embolism.
Teaching Pearls
- Always record FiO₂ with the ABG.
- VBG useful for acid–base trend; ABG required for oxygenation.
- Lactate guides sepsis resuscitation.
- Correct AG for low albumin: each 10 g/L drop ↓ AG by ~2.5–3.
Related topics: Metabolic Acidosis | ABG Analysis
