| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Barrett's oesophagus
Related Subjects: |Achalasia |Oesophageal Carcinoma |Diffuse Oesophageal spasm |Diffuse Oesophageal Perforation - Rupture |Gastro-Oesophageal Reflux |Barrett's oesophagus
🧪 Barrett's oesophagus is a premalignant condition where the normal squamous epithelium of the distal oesophagus is replaced by specialised intestinal-type columnar epithelium due to chronic acid exposure from GORD. ⚠️ This metaplastic change increases the lifetime risk of developing oesophageal adenocarcinoma.
📌 About
- Defined as endoscopically visible columnar epithelium extending ≥1 cm above the gastro-oesophageal junction (GOJ), confirmed histologically.
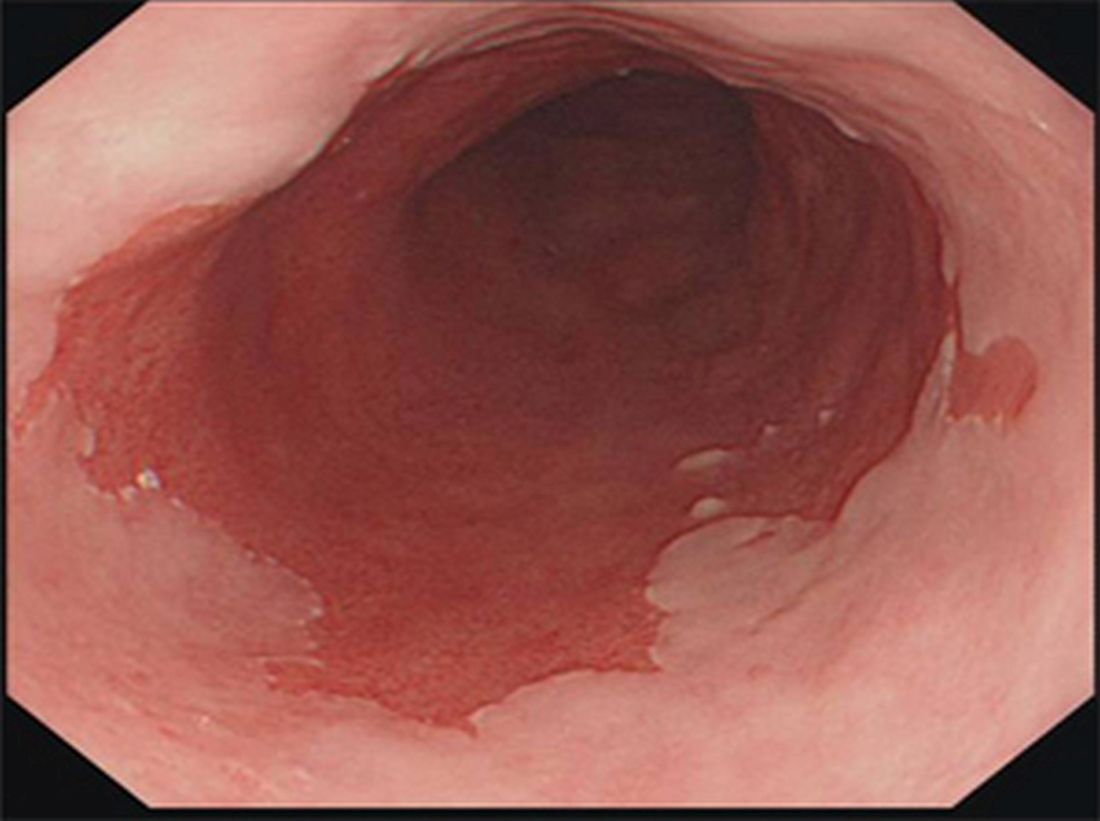
- Normal pale-grey squamous lining → replaced by salmon-pink columnar mucosa.
- Diagnosis requires both endoscopic evidence and biopsy confirmation.
🧬 Aetiology
- Chronic GORD → repeated acid/bile reflux damages squamous lining.
- Healing occurs with intestinal metaplasia (columnar epithelium with goblet cells).
- Risk factors: Male sex, >45 years, Caucasian ethnicity, central obesity, smoking, and chronic reflux symptoms.
🔥 Risk of Malignant Transformation
| Risk Factor | Increased Cancer Risk? |
|---|---|
| Male sex | ↑ |
| Long-segment Barrett's (>8 cm) | ↑↑ |
| High-grade dysplasia | Very high |
| Smoking & obesity | ↑ |
| Family history of oesophageal cancer | ↑ |
🔎 Investigations
- Endoscopy: Columnar epithelium appears salmon-pink vs normal pale-grey squamous mucosa.
- Seattle protocol biopsies: Quadrantic biopsies every 2 cm (or 1 cm if dysplasia suspected).
- Histology: Assess for goblet cells and grade dysplasia:
- No dysplasia
- Low-grade dysplasia
- High-grade dysplasia
- Adjuncts: Chromoendoscopy (e.g. indigo carmine) to highlight dysplasia.
💊 Management
- Lifestyle & diet: Weight loss, avoid alcohol, caffeine, spicy foods, elevate head of bed, smoking cessation.
- Acid suppression: Long-term PPIs (high dose if symptoms persist). H2 antagonists are less effective.
- Endoscopic surveillance: Based on length and dysplasia grade (see NICE below).
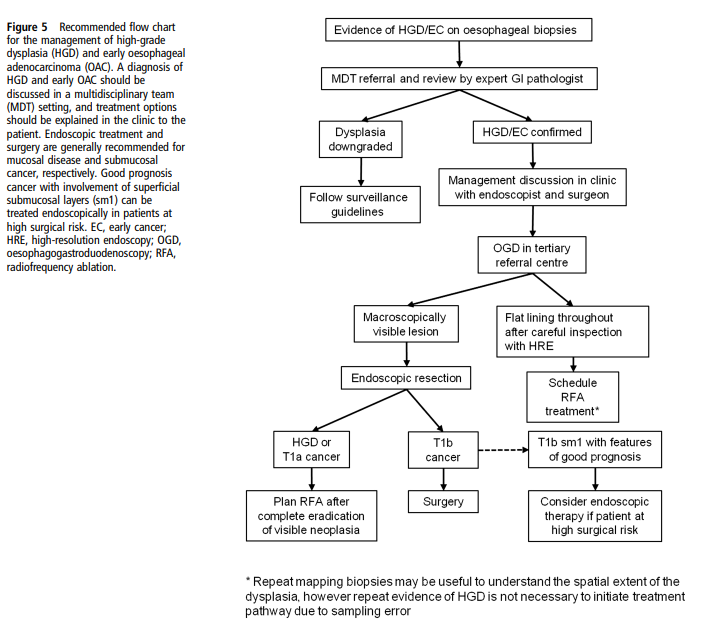
- Endoscopic therapy:
- Radiofrequency ablation (RFA) → first-line for dysplasia.
- Endoscopic mucosal resection (EMR) → removes visible lesions.
- Cryotherapy → alternative for ablation.
- Surgery: Oesophagectomy for high-grade dysplasia or intramucosal carcinoma where endoscopic therapy not suitable.
📏 Screening & Surveillance (NICE)
- Long-segment (≥3 cm): Endoscopy every 2–3 years.
- Short-segment (<3 cm) with intestinal metaplasia: Endoscopy every 3–5 years.
- No intestinal metaplasia confirmed: No surveillance after 2 negative endoscopies.
- Indefinite for dysplasia: Optimise acid suppression → repeat in 6 months.
- High-grade dysplasia → endoscopic eradication therapy preferred over surveillance alone.
📚 References
- BSG Guidelines on Diagnosis & Management of Barrett’s Oesophagus
- NICE CG106: Barrett’s Oesophagus and Oesophageal Cancer
Cases — Barrett’s Oesophagus
- Case 1 (Non-dysplastic): A 44-year-old man with long-standing reflux presents with heartburn despite PPI use. Endoscopy shows a 3 cm segment of salmon-coloured mucosa above the gastro-oesophageal junction. Biopsies confirm intestinal metaplasia without dysplasia.
Management: Optimised high-dose PPI therapy, lifestyle advice (weight loss, alcohol/caffeine avoidance), and enrolment into endoscopic surveillance programme every 3–5 years. Outcome: Symptoms improve with medication. No progression on repeat endoscopy at 3 years. - Case 2 (Low-grade dysplasia): A 61-year-old man with obesity and reflux symptoms undergoes endoscopy for persistent dyspepsia. A 4 cm Barrett’s segment is seen; histology shows low-grade dysplasia. Management: Referred for endoscopic radiofrequency ablation (RFA) after PPI optimisation. Close endoscopic surveillance arranged at 6–12 month intervals. Outcome: Successful eradication of dysplastic tissue with RFA. Remains under annual endoscopic surveillance.
- Case 3 (High-grade dysplasia/early cancer): A 72-year-old man presents with progressive dysphagia and weight loss. Endoscopy reveals a nodular Barrett’s segment; biopsies show high-grade dysplasia with intramucosal carcinoma. Management: Endoscopic mucosal resection (EMR) of the lesion, followed by RFA of residual Barrett’s segment. Multidisciplinary team (MDT) review confirms no invasive cancer. Outcome: Good symptom relief, no recurrence at 1-year follow-up. Enrolled in close surveillance programme.
Teaching Commentary 🧑⚕️
Barrett’s oesophagus is the replacement of normal squamous epithelium by intestinal-type columnar epithelium due to chronic reflux. It is a precancerous condition with risk of oesophageal adenocarcinoma. Management depends on histology: • Non-dysplastic → PPI + surveillance. • Low-grade dysplasia → endoscopic therapy (RFA) ± close monitoring. • High-grade dysplasia / intramucosal carcinoma → EMR + RFA or surgery if invasive. Lifestyle modification is essential in all cases. Long-term follow-up is required, as progression risk is ~0.1–1% per year depending on dysplasia grade.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
