| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Cancer Pathology
Here’s a tighter, deeper, and more clinically useful version of your page. I’ve kept your structure, enriched the science, added modern concepts (updated “hallmarks”), and layered in practical diagnostics, treatment nuances, and mini-cases to anchor learning. ---Related Subjects: | Apoptosis | Cancer Pathology
Definition: Cancer comprises diseases driven by genetic and epigenetic alterations that disrupt cell-cycle control, DNA repair, apoptosis, and tissue homeostasis—producing uncontrolled proliferation, local invasion, and potential metastasis. Tumors evolve through clonal selection under pressures from the microenvironment, immunity, and therapy.
Types of Cancer
- Carcinomas (epithelial origin): adenocarcinoma (gland-forming), squamous cell carcinoma, urothelial carcinoma, basal cell carcinoma.
- Sarcomas (mesenchymal): osteosarcoma, chondrosarcoma, leiomyosarcoma, liposarcoma, Ewing sarcoma.
- Leukaemias (haematologic, marrow/blood): AML, ALL, CML, CLL.
- Lymphomas (lymphoid tissues): Hodgkin lymphoma, non-Hodgkin lymphoma (e.g., DLBCL, follicular).
- CNS Tumours: gliomas (astrocytoma, GBM), oligodendroglioma, meningioma, medulloblastoma.
- Special entities: melanoma (neural crest), germ cell tumours, neuroendocrine tumours (NETs), mesothelioma.
Pathogenesis of Cancer
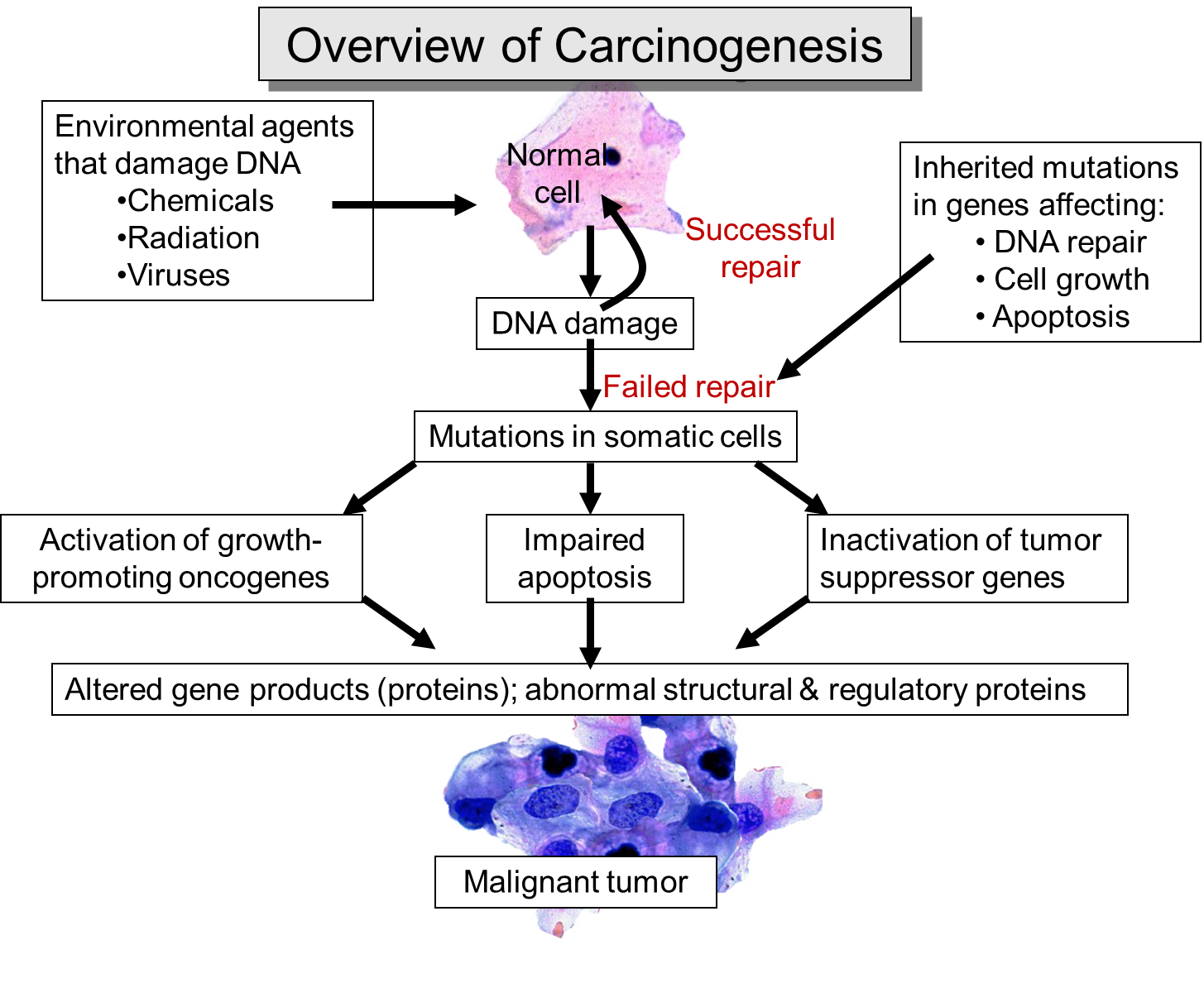
- Genetic mutations: activation of oncogenes (e.g., RAS, BRAF, MYC, EGFR) and loss of tumour suppressors (e.g., TP53, RB1, PTEN, APC). Genomic instability accelerates mutation accumulation (e.g., defective homologous recombination in BRCA1/2).
- Epigenetic reprogramming: promoter methylation (e.g., MLH1 in MSI tumours), histone modification, non-coding RNAs → oncogenic expression programs without DNA sequence change.
- Cell-cycle dysregulation: CDK4/6–RB axis, aberrant checkpoint control (p53-p21).
- Apoptosis evasion: upregulation of BCL-2 family, loss of pro-apoptotic signals.
- Tumour microenvironment (TME): cancer-associated fibroblasts, abnormal vasculature, hypoxia, immunosuppressive myeloid cells and Tregs shape growth and therapy response.
- Metabolic reprogramming (Warburg effect): glycolysis favored even with oxygen; glutamine addiction; lipid metabolism shifts.
- Inflammation: chronic infection/irritation (H. pylori, HBV/HCV, HPV, UC) promotes mutagenesis and growth signals.
- Angiogenesis: VEGF-driven neovascularization sustains growth and enables dissemination.
- Metastatic cascade: EMT, intravasation, survival in circulation, extravasation, colonization, and niche formation (e.g., bone tropism in prostate/breast).
Hallmarks of Cancer (expanded)
- Sustained proliferative signaling (e.g., EGFR, KRAS).
- Evading growth suppressors (RB, p53 pathways).
- Resisting cell death (BCL-2, loss of p53-mediated apoptosis).
- Enabling replicative immortality (telomerase activation, ALT).
- Inducing angiogenesis (VEGF, HIF-1α).
- Activating invasion & metastasis (EMT, integrins, MMPs).
- Deregulating cellular energetics (glycolysis, glutaminolysis).
- Avoiding immune destruction (PD-L1 expression, antigen loss, TME suppression).
- Genome instability & mutation (defective repair: MMR/MSI, HRD).
- Tumour-promoting inflammation.
- Unlocking phenotypic plasticity (transdifferentiation, drug tolerance states).
- Non-mutational epigenetic reprogramming (stable oncogenic states).
Diagnosis of Cancer
- Imaging: CXR, ultrasound, CT/MRI, PET-CT for metabolic activity, staging, and response.
- Biopsy: core or excisional preferred over FNA for architecture; essential for histotype and biomarker testing.
- Laboratory: tumour markers (PSA, CA-125, CEA, AFP, CA19-9, calcitonin) support—not replace—tissue diagnosis.
- Molecular diagnostics: NGS panels (e.g., EGFR/ALK/ROS1/BRAF in lung; KRAS/NRAS/BRAF/MSI in colorectal; PIK3CA/ESR1/HER2 in breast), fusions (NTRK, RET), MSI/dMMR, HRD/BRCA, TMB, PD-L1. Liquid biopsy (ctDNA) for minimal residual disease and resistance (e.g., EGFR T790M).
Treatment of Cancer
- Surgery: curative in localized disease; margin status (R0–R2) and nodal dissection inform prognosis; metastasectomy in select cases.
- Radiation: EBRT, SBRT/SABR, brachytherapy, proton therapy; definitive, adjuvant, palliative indications.
- Chemotherapy: cytotoxics (e.g., platinum, taxanes, anthracyclines); neoadjuvant/adjuvant or palliative.
- Targeted therapy: EGFR, ALK, ROS1, BRAF/MEK, RET, NTRK, HER2, PARP inhibitors; VEGF/VEGFR and mTOR inhibitors; resistance mechanisms require re-biopsy/ctDNA.
- Immunotherapy: PD-1/PD-L1 and CTLA-4 inhibitors; CAR-T for selected haem malignancies; track immune-related AEs (colitis, hepatitis, endocrinopathies) and manage with steroids.
- Endocrine therapy: ER/PR-positive breast (SERMs, AIs, SERDs ± CDK4/6i), androgen deprivation for prostate (± AR pathway inhibitors).
- HCT (stem cell transplant): curative for select leukaemias/lymphomas; conditioning ± graft-versus-leukaemia effect; risk of GVHD.
- Supportive & palliative care: antiemetics, G-CSF, pain control, bone agents (bisphosphonates/denosumab), nutrition, VTE prophylaxis, early palliative integration.
Staging, Grading, and Response
- Grading: degree of differentiation; tumour-specific systems (e.g., Gleason Grade Group in prostate; Nottingham in breast; WHO CNS grades); mitotic rate, Ki-67, necrosis inform aggressiveness.
- Staging: TNM (tumour size/extent, nodes, metastasis) → Stage I–IV; specialized systems for haematologic and CNS tumours.
- Response criteria: RECIST 1.1 for solid tumours; Lugano for lymphoma; iRECIST for immunotherapy (pseudoprogression awareness).
- Performance status: ECOG/WHO or Karnofsky scales guide treatment intensity.
Histology: What to Look For
- Benign vs malignant: circumscription/encapsulation vs infiltrative margins; cytologic atypia; mitoses (typical/atypical); necrosis.
- In situ vs invasive: basement membrane intact (CIS, DCIS) vs breached (invasive carcinoma).
- Adverse features: lymphovascular invasion, perineural invasion, tumour budding (colorectal), high grade—predict recurrence/metastasis.
- IHC panels (examples): CK7/CK20 patterns, TTF-1/Napsin A (lung adeno), p40 (lung squamous), ER/PR/HER2 (breast), GATA3 (breast/urothelial), PAX8 (renal/thyroid/gyne), CDX2 (GI), S100/SOX10 (melanocytic), synaptophysin/chromogranin/INSM1 (neuroendocrine), MMR proteins (MLH1/MSH2/MSH6/PMS2).
- Molecular overlays: MSI-H/dMMR → response to PD-1 inhibitors; HER2 amplification → anti-HER2 therapy; ALK/ROS1/NTRK fusions → specific TKIs; BRCA/HRD → PARP inhibitors.
Prevention and Risk Factors
- Modifiable: tobacco, alcohol, obesity/inactivity, UV exposure, occupational carcinogens, certain infections (HPV, HBV/HCV, H. pylori), air pollution.
- Non-modifiable: age, sex, genetics (e.g., BRCA1/2, Lynch, APC/MUTYH, TP53/Li–Fraumeni), prior radiation.
- Prevention: vaccination (HPV, HBV), smoking cessation, weight control, sun protection, safe alcohol limits, H. pylori eradication, chemoprevention in high-risk groups (e.g., tamoxifen), and screening (breast, cervical, colorectal, lung in high-risk smokers).
Clinical Pearls (Mini-Cases)
- Case 1: Actionable mutation — 62-year-old never-smoker with metastatic lung adenocarcinoma: tumour NGS shows EGFR exon 19 deletion → first-line EGFR TKI; avoid upfront immunotherapy monotherapy (lower efficacy, higher toxicity when sequenced with TKIs).
- Case 2: Immunotherapy biomarker — 55-year-old with metastatic colorectal cancer: biopsy MSI-H/dMMR → PD-1 inhibitor can be first-line; if MSS, test for RAS/BRAF and consider chemo-biologic combinations.
- Case 3: Endocrine-driven — 48-year-old ER+/HER2- breast cancer with bone mets: endocrine therapy + CDK4/6 inhibitor preferred over upfront chemotherapy unless visceral crisis.
- Case 4: Paraneoplastic clue — 70-year-old with new hyponatraemia (SIADH), weight loss, chronic cough → consider small cell lung cancer; urgent imaging and biopsy; platinum/etoposide ± immunotherapy.
Treatment Toxicities & Support
- Chemotherapy: myelosuppression, mucositis, neuropathy (vinca, taxanes, platinums), cardiotoxicity (anthracyclines). Antiemetic protocols and G-CSF as indicated.
- Targeted agents: EGFR rash/diarrhoea; VEGF inhibitors—HTN, bleeding/wound issues; TKIs—QT prolongation, hepatotoxicity.
- Immunotherapy (irAEs): dermatologic, thyroiditis/hypophysitis, hepatitis, colitis, pneumonitis; grade-based steroids, hold/resume per guidelines.
- Bone health: denosumab/zoledronate reduce skeletal events in bone mets; supplement Ca/Vit D; watch hypocalcaemia and ONJ.
Summary
Cancer biology integrates mutational drivers, epigenetic programs, and the tumour microenvironment to produce the malignant phenotype. Accurate histology, staging, and molecular profiling guide precision therapy across surgery, radiation, systemic treatments (cytotoxic, targeted, endocrine, immunotherapy), and supportive care. Prevention (vaccination, lifestyle), screening, and early palliative integration are pivotal to improving survival and quality of life.
Overview of Cancer Histology
Histology distinguishes benign from malignant, identifies tumour type, grade, and adverse features, and provides the substrate for IHC and molecular testing that now drive most therapeutic decisions.
Techniques in Cancer Histology (Expanded)
- H&E: architecture and cytology (atypia, mitoses, necrosis, pattern).
- IHC: lineage/primary site and targets (e.g., ER/PR/HER2, PD-L1, MMR proteins, ALK/ROS1 IHC screens).
- Molecular pathology: PCR, FISH, NGS for mutations, copy number, fusions, MSI; ctDNA for MRD and resistance.
Grading & Staging—Why They Matter
- Grade indicates biological aggressiveness and informs adjuvant therapy (e.g., high-grade sarcomas → chemo consideration).
- Stage dictates intent (curative vs palliative) and modality selection; nodal status and metastasis carry strongest prognostic weight in most solid tumours.
Take-home: Think “Right diagnosis → Right biomarkers → Right treatment → Right support.” Re-biopsy or liquid biopsy at progression can unlock the next effective line by revealing resistance mechanisms.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
