| Download the amazing global Makindo app: Android | Apple | |
|---|---|
| MEDICAL DISCLAIMER: Educational use only. Not for diagnosis or management. See below for full disclaimer. |
Red Blood Cell Maturation
Related Subjects: |Red blood cells |White blood cells |Lymphocytes |Platelets |Cryoprecipitate |Fresh Frozen Plasma |d Blood Cell Maturation
🩸 About
Blood cells are continuously produced through a tightly regulated process called haematopoiesis. This occurs primarily in the bone marrow — the soft, vascular, spongy tissue within bones such as the sternum, pelvis, and vertebrae — responsible for generating about 95% of all circulating blood cells. The marrow serves as both a factory and a sanctuary: it provides the physical niche, molecular signals, and stem cells required to sustain life-long blood cell renewal.
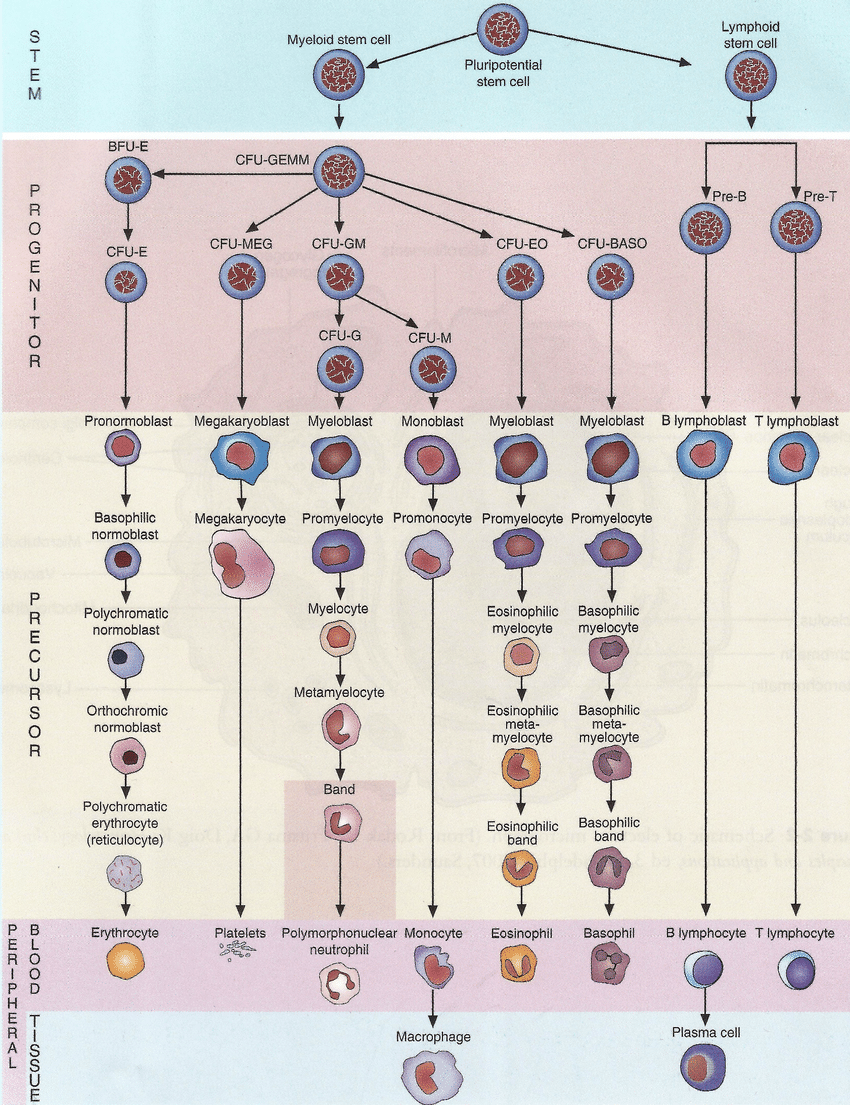
🧬 Formation of Blood Cells (Haematopoiesis)
- Haematopoiesis is the process by which haematopoietic stem cells (HSCs) give rise to all mature blood cells. It balances cell production with cell loss to maintain homeostasis.
- HSCs are rare (<1 in 104 bone marrow cells) and have two key properties:
- Self-renewal: The ability to replicate without losing potency.
- Multipotency: The capacity to differentiate into all blood lineages.
- HSCs reside in the haematopoietic niche, composed of osteoblasts, endothelial cells, mesenchymal stromal cells, and extracellular matrix proteins that regulate their proliferation and quiescence.
- During embryonic life, blood formation transitions through distinct sites:
- Yolk sac (weeks 3–8): Primitive erythropoiesis.
- Liver and spleen (weeks 6–30): Fetal haematopoiesis.
- Bone marrow (from week 30 onward): Becomes the dominant lifelong site.
🧭 Differentiation Pathways: Myeloid and Lymphoid Lineages
From the multipotent HSC arise two progenitor lines — myeloid and lymphoid — which further specialise into mature blood cells.
- Myeloid Lineage:
- Erythropoiesis: The production of red blood cells (erythrocytes) begins with the differentiation of HSCs into proerythroblasts, eventually forming enucleated erythrocytes. This process is driven by erythropoietin (EPO), secreted by the kidney in response to hypoxia.
- Thrombopoiesis: Platelets (thrombocytes) originate from megakaryocytes, large marrow cells whose cytoplasmic fragments enter the bloodstream. Regulated by thrombopoietin (TPO) from the liver and kidneys.
- Granulopoiesis and Monopoiesis: Produce neutrophils, eosinophils, basophils (granulocytes) and monocytes. Monocytes later differentiate into macrophages and dendritic cells in tissues — central to phagocytosis and antigen presentation.
- Lymphoid Lineage:
- Lymphopoiesis: Generates lymphocytes:
- B cells: Mature in bone marrow, responsible for antibody production.
- T cells: Mature in the thymus; mediate cell-based immunity (cytotoxic, helper, regulatory functions).
- Natural Killer (NK) cells: Provide innate defence against viral and malignant cells.
- Lymphopoiesis: Generates lymphocytes:
🧪 Regulation of Haematopoiesis
Haematopoiesis is dynamically regulated by an intricate network of cytokines, growth factors, transcription factors, and local microenvironmental cues. It adapts to physiological demands (e.g. infection, hypoxia, blood loss) and can expand several-fold during stress (“stress haematopoiesis”).
- Key Cytokines and Growth Factors:
- Erythropoietin (EPO): Stimulates erythroid progenitors → ↑ RBCs in response to hypoxia.
- Thrombopoietin (TPO): Regulates megakaryocyte proliferation and platelet production.
- Colony-Stimulating Factors (CSFs):
- G-CSF: Drives neutrophil production.
- GM-CSF: Stimulates granulocytes and monocytes.
- Interleukins (IL-3, IL-5, IL-7): Support differentiation of specific lineages (IL-7 for lymphopoiesis).
- Transcriptional Regulation:
Lineage commitment is orchestrated by transcription factors such as:
- GATA-1: Erythroid and megakaryocyte development.
- PU.1: Myeloid and lymphoid differentiation.
- RUNX1, TAL1, and C/EBPα: Coordinate stem cell fate and maturation.
- Microenvironmental Signals: The bone marrow niche provides oxygen tension gradients, stromal cell adhesion, and paracrine signals (CXCL12/CXCR4 axis) to control HSC retention and migration.
🧫 Clinical Relevance
- Anaemia: Reduced erythropoiesis due to iron deficiency, bone marrow failure, or chronic disease.
- Leukaemia: Clonal proliferation of immature cells disrupting normal haematopoiesis — classified as acute or chronic, myeloid or lymphoid.
- Thrombocytopenia: Impaired platelet formation or destruction → increased bleeding risk.
- Bone Marrow Failure Syndromes: Aplastic anaemia, myelodysplastic syndromes (MDS), or secondary suppression (e.g. chemotherapy, radiation).
- Therapeutic Applications:
- Bone marrow and stem cell transplantation: Restore haematopoietic function after myeloablation.
- Recombinant growth factors: EPO, G-CSF, and TPO mimetics used clinically to stimulate blood cell production.
- Gene therapy: Emerging strategy for congenital marrow disorders (e.g. sickle cell disease, thalassaemia).
🩺 Summary and Integration
Haematopoiesis represents a balance between proliferation, differentiation, and apoptosis within a highly specialised microenvironment. Through continuous renewal, it ensures adequate oxygen delivery (erythrocytes), immune defence (leucocytes), and haemostasis (platelets). Disruption of this process underlies most haematological diseases. Modern therapies — from hematopoietic stem cell transplantation to targeted cytokine modulation — are built upon understanding this elegant biological system.
💡 Teaching Tip: Think of the bone marrow as a “biological orchestra.” Stem cells are the musicians, cytokines the conductors, and the bone marrow niche the concert hall — all working in synchrony to maintain life’s most vital fluid: blood.
Categories
- A Level
- About
- Acute Medicine
- Anaesthetics and Critical Care
- Anatomy
- Anatomy and Physiology
- Biochemistry
- Book
- Cardiology
- Collections
- CompSci
- Crib Sheets
- Crib sheets
- Dental
- Dermatology
- Differentials
- Drugs
- ENT
- Education
- Electrocardiogram
- Embryology
- Emergency Medicine
- Endocrinology
- Ethics
- Foundation Doctors
- GCSE
- Gastroenterology
- General Practice
- Genetics
- Geriatric Medicine
- Guidelines
- Gynaecology
- Haematology
- Hepatology
- Immunology
- Infectious Diseases
- Infographic
- Investigations
- Lists
- Mandatory Training
- Medical Students
- Microbiology
- Nephrology
- Neurology
- Neurosurgery
- Nutrition
- OSCE
- OSCEs
- Obstetrics
- Obstetrics Gynaecology
- Oncology
- Ophthalmology
- Oral Medicine and Dentistry
- Orthopaedics
- Paediatrics
- Palliative
- Pathology
- Pharmacology
- Physiology
- Procedures
- Psychiatry
- Public Health
- Radiology
- Renal
- Respiratory
- Resuscitation
- Revision
- Rheumatology
- Statistics and Research
- Stroke
- Surgery
- Toxicology
- Trauma and Orthopaedics
- USMLE
- Urology
- Vascular Surgery
